Heat Transfer Miscellaneous
- Match the property with their units
Property Units A. Bulk modulus 1. W/s B. Thermal conductivity 2. N/m2 C. Heat transfer coefficient 3. N/m3 D. Heat flow rate 4. W 5. W/mK 6. W/m2K
-
View Hint View Answer Discuss in Forum
NA
Correct Option: B
NA
- Thermal conductivity is lower for
-
View Hint View Answer Discuss in Forum
NA
Correct Option: B
NA
- A solid sphere 1 of radius r is placed inside a hollow, closed hemispherical surface 2 of radius 4r. The shape factor F is,
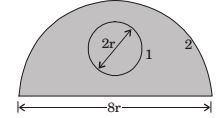
-
View Hint View Answer Discuss in Forum
f 11 + f 12 = 1
∴ f 12 = 1
f 21 A2 = f 12 A1∴ f21 = f12A1 = 1 × 4πr2 = V = 1 A2 k (1 / 2)4π(4r)2 + π(4r)2 12 Correct Option: A
f 11 + f 12 = 1
∴ f 12 = 1
f 21 A2 = f 12 A1∴ f21 = f12A1 = 1 × 4πr2 = V = 1 A2 k (1 / 2)4π(4r)2 + π(4r)2 12
- A diffuse radiation surface has
-
View Hint View Answer Discuss in Forum
Radiation intensity is independent of angle in diffuse radiation.
Correct Option: A
Radiation intensity is independent of angle in diffuse radiation.
- What is the value of the view factor for two inclined flat plates having common edge of equal length and wlth an angle of 20 degrees?
-
View Hint View Answer Discuss in Forum
F12 = F21 = 1 - sin 
θ 
2 = 1 - sin 
20° 
= 0.826 2 Correct Option: A
F12 = F21 = 1 - sin 
θ 
2 = 1 - sin 
20° 
= 0.826 2

