Nuclei
- The nuclei 6C13 and 7N14 can be described as
-
View Hint View Answer Discuss in Forum
As 6C13 and 7N14 have same no. of neutrons (13 – 6 =7 for C and 14 – 7 =7 for N),
so they are isotones.Correct Option: A
As 6C13 and 7N14 have same no. of neutrons (13 – 6 =7 for C and 14 – 7 =7 for N),
so they are isotones.
- The ratio of the radii of the nuclei 13Al27 and 52Te125 is approximately
-
View Hint View Answer Discuss in Forum
R ∝ (A)1/3
∴ RAl ∝ (27)1/3 and RTe ∝ (125)1/3∴ RAl = 3 = 6 RTe 5 10 Correct Option: A
R ∝ (A)1/3
∴ RAl ∝ (27)1/3 and RTe ∝ (125)1/3∴ RAl = 3 = 6 RTe 5 10
- A nucleus of uranium decays at rest into nuclei of thorium and helium.
Then :
-
View Hint View Answer Discuss in Forum
In an explosion a body breaks up into two pieces of unequal masses both part will have numerically equal momentum and lighter part will have more velocity.
U → Th + HeKETh P2 , KEHe = P2 2mTh 2mHe
since mHe is less so KETh will be more.Correct Option: D
In an explosion a body breaks up into two pieces of unequal masses both part will have numerically equal momentum and lighter part will have more velocity.
U → Th + HeKETh P2 , KEHe = P2 2mTh 2mHe
since mHe is less so KETh will be more.
- The Binding energy per nucleon of 3Li7 and 2He4 nuclei are 5.60 MeV and 7.06 MeV, respectively.
In the nuclear reaction 3Li7 + 1H1 → 2He4 + Q, the value of energy Q released is :
-
View Hint View Answer Discuss in Forum
BE of 2He4 = 4 × 7.06 = 28.24 MeV
BE of 7 Li = 7 × 5.60 = 39.20 MeV 3 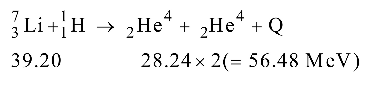
Therefore, Q = 56.48 – 39.20 = 17.28 MeV.Correct Option: D
BE of 2He4 = 4 × 7.06 = 28.24 MeV
BE of 7 Li = 7 × 5.60 = 39.20 MeV 3 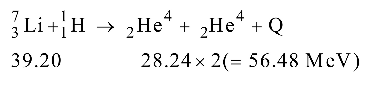
Therefore, Q = 56.48 – 39.20 = 17.28 MeV.
- A sample of radioactive element has a mass of 10 gm at an instant t=0. The approximate mass of this element in the sample after two mean lives is
-
View Hint View Answer Discuss in Forum
Using the relation for mean life.
Given : t = 2τ = 2 
1 
λ 
∴ τ = 1 
λ
Then from= 10 
1 
2 = 1.35g 2 Correct Option: B
Using the relation for mean life.
Given : t = 2τ = 2 
1 
λ 
∴ τ = 1 
λ
Then from= 10 
1 
2 = 1.35g 2

