Nuclei
- A nucleus ZXA has mass represented by M(A, Z). If Mp and Mn denote the mass of proton and neutron respectively and B.E. the binding energy in MeV, then
-
View Hint View Answer Discuss in Forum
The difference in mass of a nucleus and its constituents, ΔM, is called the mass defect and is given by
ΔM = [ZMp + (A - Z)Mn] - M
and binding energy = ΔMc2
[{ZMp + (A - Z)Mn} - M]c2Correct Option: A
The difference in mass of a nucleus and its constituents, ΔM, is called the mass defect and is given by
ΔM = [ZMp + (A - Z)Mn] - M
and binding energy = ΔMc2
[{ZMp + (A - Z)Mn} - M]c2
- If M (A; Z), Mp and Mn denote the masses of the nucleus ZXA proton and neutron respectively in units of u ( 1u = 931.5 MeV/c2) and BE represents its bonding energy in MeV, then
-
View Hint View Answer Discuss in Forum
Mass defect = ZMp + (A –Z)Mn–M(A,Z)
or, B.E c2
= ZMp + (A–Z) Mn–M(A,Z) ∴ M (A, Z) = ZMp + (A–Z)Mn – B.E c2 Correct Option: A
Mass defect = ZMp + (A –Z)Mn–M(A,Z)
or, B.E c2
= ZMp + (A–Z) Mn–M(A,Z) ∴ M (A, Z) = ZMp + (A–Z)Mn – B.E c2
- A nucleus of mass M emits a photon of frequency ν and the nucleus recoils. The recoil energy will be
-
View Hint View Answer Discuss in Forum
Momentum
Mu = E = hv c c
Recoil energy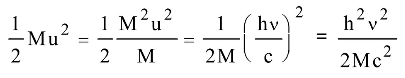
Correct Option: B
Momentum
Mu = E = hv c c
Recoil energy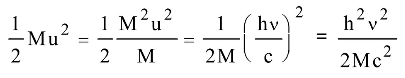
- How does the binding energy per nucleon vary with the increase in the number of nucleons?
-
View Hint View Answer Discuss in Forum
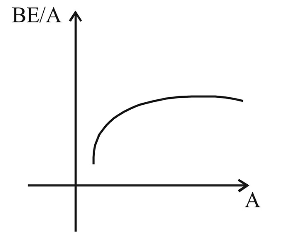
From the graph of BE/A versus mass number A it is clear that, BE/A first increases and then decreases with increase in mass number.Correct Option: D

From the graph of BE/A versus mass number A it is clear that, BE/A first increases and then decreases with increase in mass number.
- The power obtained in a reactor using U235 disintegration is 1000 kW. The mass decay of U235 per hour is
-
View Hint View Answer Discuss in Forum
E = mc2
m = E c2
So, mass decay per seconddm = 1 dE = 1 (Power in watt) dt c2 dt c2 = 1 × 1000 × 103 (3 × 108)2
and mass decay per hour= dm × 60 × 60 dt = 1 × 106 × 3600 (3 × 108)2
= 4 × 10–8 kg = 40 micro gramCorrect Option: C
E = mc2
m = E c2
So, mass decay per seconddm = 1 dE = 1 (Power in watt) dt c2 dt c2 = 1 × 1000 × 103 (3 × 108)2
and mass decay per hour= dm × 60 × 60 dt = 1 × 106 × 3600 (3 × 108)2
= 4 × 10–8 kg = 40 micro gram

