Nuclei
- The mass of a 3Li7 nucleus is 0.042 u less than the sum of the masses of all its nucleons. The binding energy per nucleon of 3Li7 nucleus is nearly
-
View Hint View Answer Discuss in Forum
B.E. = 0.042 × 931 ≃ 42 MeV
Number of nucleons in7 Li is 7. 3
∴ B.E./ nucleon = 42/7 = 6 MeV ≃ 5.6 MeVCorrect Option: B
B.E. = 0.042 × 931 ≃ 42 MeV
Number of nucleons in7 Li is 7. 3
∴ B.E./ nucleon = 42/7 = 6 MeV ≃ 5.6 MeV
- Fusion reaction takes place at high temperature because
-
View Hint View Answer Discuss in Forum
When the coulomb repulsion between the nuclei is overcome then nuclear fusion reaction takes place. This is possible when temperature is too high.
Correct Option: C
When the coulomb repulsion between the nuclei is overcome then nuclear fusion reaction takes place. This is possible when temperature is too high.
- A nucleus of mass M emits a photon of frequency ν and the nucleus recoils. The recoil energy will be
-
View Hint View Answer Discuss in Forum
Momentum
Mu = E = hv c c
Recoil energy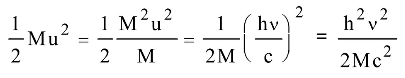
Correct Option: B
Momentum
Mu = E = hv c c
Recoil energy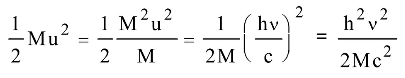
- The power obtained in a reactor using U235 disintegration is 1000 kW. The mass decay of U235 per hour is
-
View Hint View Answer Discuss in Forum
E = mc2
m = E c2
So, mass decay per seconddm = 1 dE = 1 (Power in watt) dt c2 dt c2 = 1 × 1000 × 103 (3 × 108)2
and mass decay per hour= dm × 60 × 60 dt = 1 × 106 × 3600 (3 × 108)2
= 4 × 10–8 kg = 40 micro gramCorrect Option: C
E = mc2
m = E c2
So, mass decay per seconddm = 1 dE = 1 (Power in watt) dt c2 dt c2 = 1 × 1000 × 103 (3 × 108)2
and mass decay per hour= dm × 60 × 60 dt = 1 × 106 × 3600 (3 × 108)2
= 4 × 10–8 kg = 40 micro gram
- How does the binding energy per nucleon vary with the increase in the number of nucleons?
-
View Hint View Answer Discuss in Forum
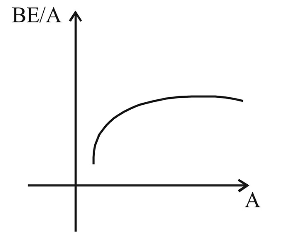
From the graph of BE/A versus mass number A it is clear that, BE/A first increases and then decreases with increase in mass number.Correct Option: D

From the graph of BE/A versus mass number A it is clear that, BE/A first increases and then decreases with increase in mass number.

