Dual Nature of Radiation and Matter
- A photoelectric surface is illuminated successively by monochromatic light of wavelength λ and λ/2. If the maximum kinetic energy of the emitted photoelectrons in the second case is 3 times that in the first case, the work function of the surface of the material is :
(h = Planck's constant, c = speed of light)
-
View Hint View Answer Discuss in Forum
Photoelectric equations
Ek1max = hc - φ ....(i) λ and Ek2max = hc - φ λ/2 Ek2max = 2hc - φ ....(ii) λ
From question, Ek2max = 3Ek1max Multiplying equation (i) by 3 3Ek1max = 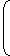
hc - φ 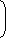
....(iii) λ
From equation (ii) and (iii)3hc - 3φ = 2hc - φ λ λ ∴ φ (work function) = hc 2λ Correct Option: D
Photoelectric equations
Ek1max = hc - φ ....(i) λ and Ek2max = hc - φ λ/2 Ek2max = 2hc - φ ....(ii) λ
From question, Ek2max = 3Ek1max Multiplying equation (i) by 3 3Ek1max = 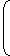
hc - φ 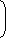
....(iii) λ
From equation (ii) and (iii)3hc - 3φ = 2hc - φ λ λ ∴ φ (work function) = hc 2λ
- A certain metallic surface is illuminated with monochromatic light of wavelength λ. The stopping potential for photo-electric current for this light is 3V0. If the same surface is illuminated with light of wavelength 2λ, the stopping potential is V0. The threshold wavelength for this surface for photo-electric effect is
-
View Hint View Answer Discuss in Forum
As we know,
eVs = hc - ψ λ 3eV0 = hc - ψ ....(i) λ eV0 = hc - ψ ....(ii) λ 3eV0 = 3hc - 3ψ ....(iii) 2λ
Multiplying eqn. (2) by (3) and subtracting it from eqn (1)ψ = hc 4λ
So,threshold wavelength,λth = hc = hc = 4λ λ hc/4λ Correct Option: A
As we know,
eVs = hc - ψ λ 3eV0 = hc - ψ ....(i) λ eV0 = hc - ψ ....(ii) λ 3eV0 = 3hc - 3ψ ....(iii) 2λ
Multiplying eqn. (2) by (3) and subtracting it from eqn (1)ψ = hc 4λ
So,threshold wavelength,λth = hc = hc = 4λ λ hc/4λ
- Light of wavelength 500 nm is incident on a metal with work function 2.28 eV. The wavelength of the emitted electron is:
-
View Hint View Answer Discuss in Forum
Given : Work function φ of metal = 2.28 eV
Wavelength of light λ = 500 nm = 500 × 10–9 mKEmax = hc - φ λ KEmax = 6.6 × 10-34 × 3 × 108 - 2.28 5 × 10-7 × 1.6 × 10-19
KEmax = 2.48 – 2.28 = 0.2 evλmin = h = h p √2 m(KE)max = 20 × 10-34 3 √2 × 9 × 10-31 × 0.2 × 1.6 × 10-19 λmin = 25 × 10-9 9
= 2.80 × 10–9 nm
∴ λ ≥ 2.8 × 10–9 mCorrect Option: B
Given : Work function φ of metal = 2.28 eV
Wavelength of light λ = 500 nm = 500 × 10–9 mKEmax = hc - φ λ KEmax = 6.6 × 10-34 × 3 × 108 - 2.28 5 × 10-7 × 1.6 × 10-19
KEmax = 2.48 – 2.28 = 0.2 evλmin = h = h p √2 m(KE)max = 20 × 10-34 3 √2 × 9 × 10-31 × 0.2 × 1.6 × 10-19 λmin = 25 × 10-9 9
= 2.80 × 10–9 nm
∴ λ ≥ 2.8 × 10–9 m
- Photoelectric emmision occurs only when the incident light has more than a certain minimum
-
View Hint View Answer Discuss in Forum
For occurence of photoelectric effect, the incident light should have frequency more than a certain minimum which is called the threshold frequency (v0).
We have,1 mv2 = hv - hv0 2
For photoelectric effect emission ν > ν0
where ν is the frequency of the incident light.Correct Option: D
For occurence of photoelectric effect, the incident light should have frequency more than a certain minimum which is called the threshold frequency (v0).
We have,1 mv2 = hv - hv0 2
For photoelectric effect emission ν > ν0
where ν is the frequency of the incident light.
- When the energy of the incident radiation is incredased by 20%, the kinetic energy of the photoelectrons emitted from a metal surface increased from 0.5 eV to 0.8 eV. The work function of the metal is :
-
View Hint View Answer Discuss in Forum
According to Einstein’s photoelectric equation, hν = φ0 + Kmax
We have
hν = φ0 + 0.5 ...(i)
and1.2hν = φ0 + 0.8 ...(ii)
Therefore, from above two equations φ0 = 1.0 eV.Correct Option: B
According to Einstein’s photoelectric equation, hν = φ0 + Kmax
We have
hν = φ0 + 0.5 ...(i)
and1.2hν = φ0 + 0.8 ...(ii)
Therefore, from above two equations φ0 = 1.0 eV.

