Atoms
- The total energy of an electron in the first excited state of hydrogen atom is about –3.4 eV. Its kinetic energy in this state is
-
View Hint View Answer Discuss in Forum
K.E = Z2 (13.6 eV) n2 Mechanical energy = - Z2 (13.6 eV) n2
∴ K.E. in 2nd orbital for hydrogen = – Mechanical energy= 12 (13.6) = + 3.4 eV 22 Correct Option: A
K.E = Z2 (13.6 eV) n2 Mechanical energy = - Z2 (13.6 eV) n2
∴ K.E. in 2nd orbital for hydrogen = – Mechanical energy= 12 (13.6) = + 3.4 eV 22
- Ionization potential of hydrogen atom is 13.6eV. Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12.1 eV. According to Bohr’s theory, the spectral lines emitted by hydrogen will be
-
View Hint View Answer Discuss in Forum
Energy of ground state 13.6 eV Energy of first excited state
= - 13.6 = - 3.4eV 4
Energy of second excited state= - 13.6 = - 1.5eV 9
Difference between ground state and 2nd excited state = 13.6 – 1.5 = 12.1 eV
So, electron can be excited upto 3rd orbit No. of possible transition
1 → 2, 1 → 3, 2 → 3
So, three lines are possible.Correct Option: A
Energy of ground state 13.6 eV Energy of first excited state
= - 13.6 = - 3.4eV 4
Energy of second excited state= - 13.6 = - 1.5eV 9
Difference between ground state and 2nd excited state = 13.6 – 1.5 = 12.1 eV
So, electron can be excited upto 3rd orbit No. of possible transition
1 → 2, 1 → 3, 2 → 3
So, three lines are possible.
- The total energy of electron in the ground state of hydrogen atom is – 13.6 eV. The kinetic energy of an electron in the first excited state is
-
View Hint View Answer Discuss in Forum
Energy in the first excited state
= - 13.6 n2 = - 13.6 = - 3.4eV 22
But K.E. = –(Total energy) = +3.4 eV.Correct Option: D
Energy in the first excited state
= - 13.6 n2 = - 13.6 = - 3.4eV 22
But K.E. = –(Total energy) = +3.4 eV.
- The ground state energy of hydrogen atom is 13.6eV. When its electron is in the first excited state, its excitation energy is
-
View Hint View Answer Discuss in Forum
When the electron is in first excited state (n = 2), the excitation energy is given by
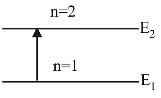
∆E = E2 – E1
We have,En = - 13.6 eV n2 ∴ E2 = - 13.6 = - 3.4eV 22
Given E1 = –13.6eV
∴ ∆ E = ( – 3.4) – ( – 13.6) = 10.2 eV.Correct Option: C
When the electron is in first excited state (n = 2), the excitation energy is given by

∆E = E2 – E1
We have,En = - 13.6 eV n2 ∴ E2 = - 13.6 = - 3.4eV 22
Given E1 = –13.6eV
∴ ∆ E = ( – 3.4) – ( – 13.6) = 10.2 eV.

