-
The ground state energy of hydrogen atom is 13.6eV. When its electron is in the first excited state, its excitation energy is
-
- 3.4 eV
- 6.8 eV
- 10.2 eV
- 0
Correct Option: C
When the electron is in first excited state (n = 2), the excitation energy is given by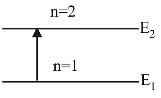
∆E = E2 – E1
We have,
| En = - | eV | n2 |
| ∴ E2 = - | = - 3.4eV | 22 |
Given E1 = –13.6eV
∴ ∆ E = ( – 3.4) – ( – 13.6) = 10.2 eV.

