Atoms
- An electron in hydrogen atom makes a transition n1 → n2 where n1 and n2 are principal quantum numbers of the two states. Assuming Bohr’s model to be valid the time period of the electron in the initial state is eight times that in the final state. The possible values of n1 and n2 are
-
View Hint View Answer Discuss in Forum
∴ T ∝ n3
T1 = 8T2 = 
n1 
3 T2 T2 n2
Hence, n1 = 2n2
Correct Option: A
∴ T ∝ n3
T1 = 8T2 = 
n1 
3 T2 T2 n2
Hence, n1 = 2n2
- An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be :
(m is the mass of the electron, R, Rydberg constant and h Planck’s constant)
-
View Hint View Answer Discuss in Forum
For emission, the wave number of the radiation is given as
1 = RZ2 
1 - 1 
λ n21 n22
R = Rydberg constant, Z = atomic number= R 
1 - 1 
12 52 = R 
1 - 1 
25 ⇒ 1 = R 24 λ 25
linear momentumP = h = h × R × 24 λ 25
(de-Broglie hypothesis)⇒ mv = 24hR ⇒ V = 24hR 25 25m Correct Option: A
For emission, the wave number of the radiation is given as
1 = RZ2 
1 - 1 
λ n21 n22
R = Rydberg constant, Z = atomic number= R 
1 - 1 
12 52 = R 
1 - 1 
25 ⇒ 1 = R 24 λ 25
linear momentumP = h = h × R × 24 λ 25
(de-Broglie hypothesis)⇒ mv = 24hR ⇒ V = 24hR 25 25m
- Electron in hydrogen atom first jumps from third excited state to second excited state and then from second excited to the first excited state. The ratio of the wavelength λ1 : λ2 emitted in the two cases is
-
View Hint View Answer Discuss in Forum
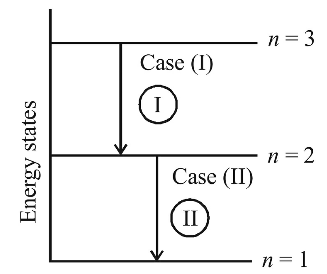
The wave number (V) of the radiation= 1 λ 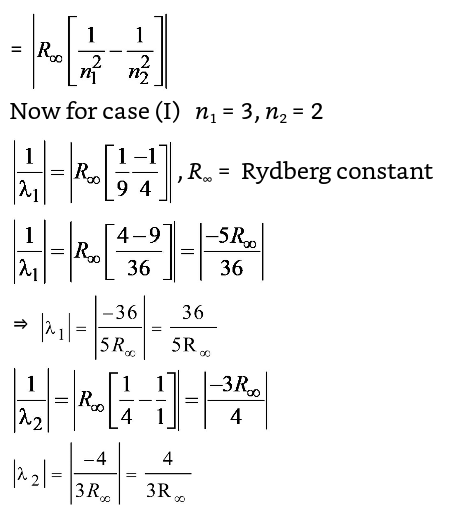
⇒ λ1 = 36 × 3R∞ λ2 5R∞ 4 λ1 = 27 λ2 5 Correct Option: C

The wave number (V) of the radiation= 1 λ 
⇒ λ1 = 36 × 3R∞ λ2 5R∞ 4 λ1 = 27 λ2 5
- The transition from the state n = 3 to n = 1 in a hydrogen like atom results in ultraviolet radiation. Infrared radiation will be obtained in the transition from :
-
View Hint View Answer Discuss in Forum
∵ The frequency of the transition
V ∝ 1 n2
when n = 1, 2, 3.Correct Option: D
∵ The frequency of the transition
V ∝ 1 n2
when n = 1, 2, 3.
- Hydrogen atom in ground state is excited by a monochromatic radiation of λ = 975 Å. Number of spectral lines in the resulting spectrum emitted will be
-
View Hint View Answer Discuss in Forum
For the λ = 975 Å
1 = R 
1 - 1 
λ n21 n22
where R is the Rydberg constant
Solving we get n2 = n = 4
(∵ n1 = 1 ground state)
Therefore number of spectral lines= n(n - 1) = 4(4 - 1) = 6 2 2 Correct Option: C
For the λ = 975 Å
1 = R 
1 - 1 
λ n21 n22
where R is the Rydberg constant
Solving we get n2 = n = 4
(∵ n1 = 1 ground state)
Therefore number of spectral lines= n(n - 1) = 4(4 - 1) = 6 2 2

