-
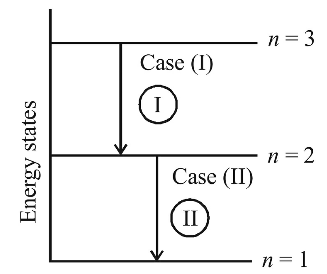
Electron in hydrogen atom first jumps from third excited state to second excited state and then from second excited to the first excited state. The ratio of the wavelength λ1 : λ2 emitted in the two cases is
-
- 7/5
- 27/20
- 27/5
- 20/7
Correct Option: C
The wave number (V) of the radiation
| = | λ |
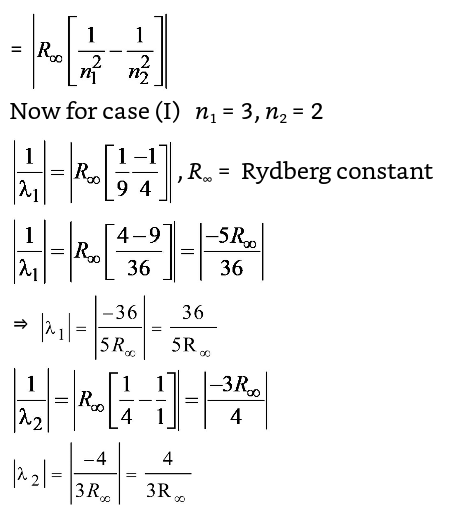
| ⇒ | = | × | ||||
| λ2 | 5R∞ | 4 |
| = | ||||
| λ2 | 5 |

