Atoms
- An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be :
(m is the mass of the electron, R, Rydberg constant and h Planck’s constant)
-
View Hint View Answer Discuss in Forum
For emission, the wave number of the radiation is given as
1 = RZ2 
1 - 1 
λ n21 n22
R = Rydberg constant, Z = atomic number= R 
1 - 1 
12 52 = R 
1 - 1 
25 ⇒ 1 = R 24 λ 25
linear momentumP = h = h × R × 24 λ 25
(de-Broglie hypothesis)⇒ mv = 24hR ⇒ V = 24hR 25 25m Correct Option: A
For emission, the wave number of the radiation is given as
1 = RZ2 
1 - 1 
λ n21 n22
R = Rydberg constant, Z = atomic number= R 
1 - 1 
12 52 = R 
1 - 1 
25 ⇒ 1 = R 24 λ 25
linear momentumP = h = h × R × 24 λ 25
(de-Broglie hypothesis)⇒ mv = 24hR ⇒ V = 24hR 25 25m
- An electron in the hydrogen atom jumps from excited state n to the ground state. The wavelength so emitted illuminates a photosensitive material having work function 2.75 eV. If the stopping potential of the photoelectron is 10 V, the value of n is
-
View Hint View Answer Discuss in Forum
KEmax = 10eV
φ = 2.75 eV
Total incident energy
E = φ + KEmax = 12.75 eV
∴ Energy is released when electron jumps from the excited state n to the ground state.
E4 – E4 = {– 0.85 – (–13.6) ev} = 12.75 eV
∴ value of n = 4Correct Option: B
KEmax = 10eV
φ = 2.75 eV
Total incident energy
E = φ + KEmax = 12.75 eV
∴ Energy is released when electron jumps from the excited state n to the ground state.
E4 – E4 = {– 0.85 – (–13.6) ev} = 12.75 eV
∴ value of n = 4
- The energy of a hydrogen atom in the ground state is – 13.6 eV. The energy of a He+ ion in the first excited state will be
-
View Hint View Answer Discuss in Forum
Energy of a H-like atom in it's nth state is given by
En = -Z2 × 13.6 eV n2
For, first excited state of He+, n = 2, Z = 2EHe+ = - 4 × 13.6 = - 13.6 eV 22 Correct Option: A
Energy of a H-like atom in it's nth state is given by
En = -Z2 × 13.6 eV n2
For, first excited state of He+, n = 2, Z = 2EHe+ = - 4 × 13.6 = - 13.6 eV 22
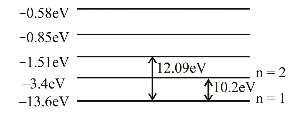
- Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr’s atomic model?
-
View Hint View Answer Discuss in Forum
Obviously, difference of 11.1eV is not possible.
Correct Option: B
Obviously, difference of 11.1eV is not possible.

- Two particles of masses m1, m2 move with initial velocities u1 and u2. On collision, one of the particles get excited to higher level, after absorbing energy ε. If final velocities of particles be v1 and v2 then we must have
-
View Hint View Answer Discuss in Forum
By law of conservation of energy,
K.Ef = K.Ei – excitation energy (ε)
or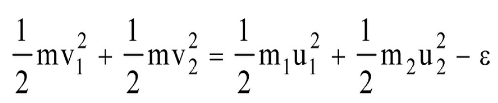
Correct Option: B
By law of conservation of energy,
K.Ef = K.Ei – excitation energy (ε)
or