Water requirements miscellaneous
Direction: Ion concentrations obtained for a groundwater sample (having pH = 8.1) are given below: 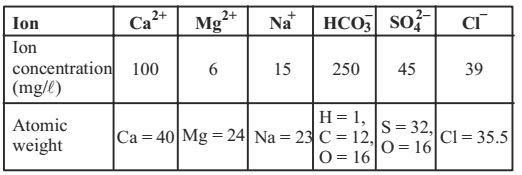
- Carbonate hardness (mg/l as CaCO3) present in the above water sample is
-
View Hint View Answer Discuss in Forum
NA
Correct Option: D
NA
- Total hardness (mg/l as CaCO3) present in the above water sample is
-
View Hint View Answer Discuss in Forum
NA
Correct Option: C
NA
Direction: Following chemical species were reported for water sample from a well: 
- Alkalinity present in the water in mg/l as CaCO3 is
-
View Hint View Answer Discuss in Forum
NA
Correct Option: C
NA
- Ultimate BOD of a river water sample is 20 mg/L. BOD rate constant (natural log) is 0.15 day-1. The respective values of BOD (in%) exerted and remaining after 7 days are:
-
View Hint View Answer Discuss in Forum
Yu = 20 mg/L
Y7 = Yu × e–kt
= 20 × e–0.15 × 7 = 7% remaining = 7 × 100 = 35% 20
% exerted = 100 – 35 = 65%Correct Option: C
Yu = 20 mg/L
Y7 = Yu × e–kt
= 20 × e–0.15 × 7 = 7% remaining = 7 × 100 = 35% 20
% exerted = 100 – 35 = 65%
- A waste water stream (flow = 2m³/s, ultimate BOD = 90 mg/l) is joining a small river (flow = 12 m³/s, ultimate BOD = 5 mg/ l). Both water streams get mixed up instantaneously. Crosssectional area of the river is 50 m². Assuming the deoxygenation rate constant, k = 0.25.day, the BOD (in mg/l) of the river water, 10 km downstream of the mixing point is
-
View Hint View Answer Discuss in Forum
BOD of mixture, Y = (2 × 90) + (12 × 5) = 17.143 g/m³ 2 × 12
KD = 0.434 × k1
= 0.434 × 25 = 0.1085Velocity of stream = 14 = 0.28 m/s 50 Time taken = 10 km = 9.921 hours 0.28 m/s
Yt = Yo (1 – 10–kt)
= 17.143 (1 – 10–0.1085 × 9.921) = 15.7 mg/lCorrect Option: C
BOD of mixture, Y = (2 × 90) + (12 × 5) = 17.143 g/m³ 2 × 12
KD = 0.434 × k1
= 0.434 × 25 = 0.1085Velocity of stream = 14 = 0.28 m/s 50 Time taken = 10 km = 9.921 hours 0.28 m/s
Yt = Yo (1 – 10–kt)
= 17.143 (1 – 10–0.1085 × 9.921) = 15.7 mg/l

