Water requirements miscellaneous
- The alkalinity and hardness of a water sample are 250 mg/l and 350 mg/l as CaCO3, respectively. The water has
-
View Hint View Answer Discuss in Forum
The lower of alkalinity and hardness is carbonate value.
This, carbonate hardness = 250 mg/l.
Non-carbonate hardness = 350 – 250 = 100 mg/lCorrect Option: D
The lower of alkalinity and hardness is carbonate value.
This, carbonate hardness = 250 mg/l.
Non-carbonate hardness = 350 – 250 = 100 mg/l
- A synthetic sample of water is prepared by adding 100 mg Kaolinite (a clay mineral), 200 mg glucose, 168 mg NaCl, 120 mg MgSO4 and 111 mg CaCl2 to 1 litre of pure water. The concentrations of total solids (TS) and fixed dissolved solids (FDS) respectively in the solution in mg/L are equal to
-
View Hint View Answer Discuss in Forum
Total solids = 100 + 200 + 168 + 120 + 111 = 699 mg/l
Fixed dissolved solids = 200 + 168 + 120 + 111 = 599 mg/l
Kaolinite is insoluble.Correct Option: A
Total solids = 100 + 200 + 168 + 120 + 111 = 699 mg/l
Fixed dissolved solids = 200 + 168 + 120 + 111 = 599 mg/l
Kaolinite is insoluble.
- If tomato juice is having pH of 4.1, the hydrogen ion concentration will be
-
View Hint View Answer Discuss in Forum
pH = – log[H+]
4.1 = – log [H+]
∴ [H+] = 7.94 × 10-5 mol/LCorrect Option: D
pH = – log[H+]
4.1 = – log [H+]
∴ [H+] = 7.94 × 10-5 mol/L
- Chlorine gas used for disinfection combines with water to form hypochlorous acid (HOCl). The HOCl ionizes to form hypochlorite (OCl–) in a reversible reaction:
HOCl ↔ H+ + OCl– (k = 2.7 × 10–8 at 20° C)
the equilibrium of which is governed by pH. the sum of HOCI and OCl– is known as free chlorine residual and HOCl is the more effective disinfectant. The 90% fraction of HOCl in the free chlorine residual is available at a pH value.
-
View Hint View Answer Discuss in Forum
In positive combination 4 – 3 – 1, MPN index = 33.
Correct MPN = MPN Value × 100 Largest volume tested = 33 × 100 = 330 100 Correct Option: D
In positive combination 4 – 3 – 1, MPN index = 33.
Correct MPN = MPN Value × 100 Largest volume tested = 33 × 100 = 330 100
- A standard multiple-tube fermentation test was conducted on a sample of water from a surface stream. The results of the analysis for the confirmed test are given below.
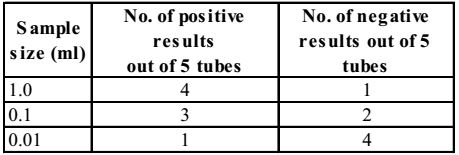
MPN Index and 95% confidence limits for combination of positive results when five tubes used per dilutions (10 ml, 1.0 ml 0.1 ml)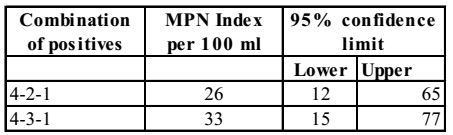
Using the above MPN Index table, the most probable number (MPN) of the sample is
-
View Hint View Answer Discuss in Forum
Q = 1 R2/3s01/2A n
n = 0.015
q = 1728 m³/day.A = π × D² = π × (0.3)² = 0.07 m² 4 4 R = A = π/4D² = D = 0.3 = 0.075 m P πD 4 4 Q = 1 R2/3s1/2 × A n = A = 1 × (0.075)2/3 × 
1 
1/2 × 0.07 P 0.015 280
= 0.0495 = 0.05 m³/s = 4320 m³/dayq = 1728 = 0.4 Q 4320
From the graph,d = 0.5 ⇒ 0.5, × 0.3 = .15 m = 150 mm D For d = 0.5, v = 0.8 D V ∴ v = 0.8 × V = 0.8 × Q = 0.8 × 0.05 = 0.8 × 714 = 0.57 m/s A 0.07 Correct Option: C
Q = 1 R2/3s01/2A n
n = 0.015
q = 1728 m³/day.A = π × D² = π × (0.3)² = 0.07 m² 4 4 R = A = π/4D² = D = 0.3 = 0.075 m P πD 4 4 Q = 1 R2/3s1/2 × A n = A = 1 × (0.075)2/3 × 
1 
1/2 × 0.07 P 0.015 280
= 0.0495 = 0.05 m³/s = 4320 m³/dayq = 1728 = 0.4 Q 4320
From the graph,d = 0.5 ⇒ 0.5, × 0.3 = .15 m = 150 mm D For d = 0.5, v = 0.8 D V ∴ v = 0.8 × V = 0.8 × Q = 0.8 × 0.05 = 0.8 × 714 = 0.57 m/s A 0.07

