Water requirements miscellaneous
- The potable water is prepared from turbid surface water by adopting the following treatment sequence.
-
View Hint View Answer Discuss in Forum
Coagulation – flocculation process is always followed by Sedimentation and then filtration. Disinfection is the last process.
Correct Option: D
Coagulation – flocculation process is always followed by Sedimentation and then filtration. Disinfection is the last process.
- The dominating microorganisms in an activated sludge process reactor are
-
View Hint View Answer Discuss in Forum
NA
Correct Option: A
NA
- An ideal horizontal flow setting basin in 3m deep having surface area 900 m². Water flows at the rate of 8000 m³ /d, at water temperature 20°C (m = 10³ kg/m.s and p = 1000 kg/m³). Assuming Stokes law to be valid, the proportion (percentage) of spherical sand particles (0.01 mm in diameter with specific gravity 2.65), that will be removed is
-
View Hint View Answer Discuss in Forum
NA
Correct Option: C
NA
- Results of a water sample analysis are as follows:

(milliequivalent weight of CaCO3 = 50 mg/meq).
Harness of the water sample in mg/L as CaCO3 is
-
View Hint View Answer Discuss in Forum
Hardness = Mg2+ × equivalent wt of CaCO3 + Ca2+ × equivalent wt of CaCO3 equivalent wt of Mg2+ equivalent wt of Ca2+ = 20 × 50 + 55 × 50 12.2 20
= 179 mg/l of CaCO3.Correct Option: C
Hardness = Mg2+ × equivalent wt of CaCO3 + Ca2+ × equivalent wt of CaCO3 equivalent wt of Mg2+ equivalent wt of Ca2+ = 20 × 50 + 55 × 50 12.2 20
= 179 mg/l of CaCO3.
- The theoretical oxygen dmenad of a 0.001 mol/L glucose solution is
-
View Hint View Answer Discuss in Forum
Glucose: C6H12O6
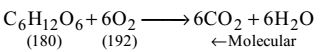
0.001 mol/L of glucose = 180 × 0.001 g/litre
= 180 mg/L glucose
From the equation 180 parts glucose need 192 parts oxygen.
∴ 180 mg/L glucose need 192 mg/L oxygenCorrect Option: B
Glucose: C6H12O6
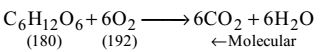
0.001 mol/L of glucose = 180 × 0.001 g/litre
= 180 mg/L glucose
From the equation 180 parts glucose need 192 parts oxygen.
∴ 180 mg/L glucose need 192 mg/L oxygen

