Refrigeration and Air-conditioning Miscellaneous
Direction: A refrigerator based on ideal vapour compression cycle operates between the temperature limits of –20°C and 40°C. The refrigerant enters the condenser as saturated vapour and leaves as saturated liquid. The enthalpy and entropy values for saturated liquid and vapour at these temperatures are given in the table below.
| T | hf | hg | sf | sg |
| (°C) | (kJ/kg) | (kJ/kg) | (kJ/kgK) | (kJ/kgK) |
| –20 | 20 | 180 | 0.07 | 0.7366 |
| 40 | 80 | 200 | 0.3 | 0.67 |
- If refrigerant circulation rate is 0.025 kg/s, the refrigeration effect is equal to
-
View Hint View Answer Discuss in Forum
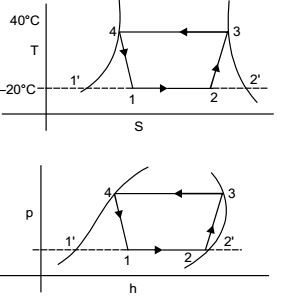
h1 = h4 = 80 kJ/kg , s3 = 0.67
h3 = 200 kJ/kg , s2 = 0.67
h1' = 20 kJ/kg , s2' = 0.7366
h2' = 20 kJ/kg , s1' = 0.07
s2 = s1' + x(s2' - s1')
= 0.07 + x + (0.7366 – 0.07)
⇒ 0.67 = 0.07 + 0.6666 x⇒ x = 0.67 - 0.07 = 0.90 0.6666
h2 = h1' + x(h2' - h1')
= 20 + 0.9(180 – 20)
= 20 + 0.9 × 160 = 165 kJ/kg
Refrigerating effect per kg = h2 – h1 = 164 – 80 = 84 kJ/kg
Hence refrigerating effect for 0.025 kg/sec circulation = 84 × 0.025 = 2.1 kWCorrect Option: A

h1 = h4 = 80 kJ/kg , s3 = 0.67
h3 = 200 kJ/kg , s2 = 0.67
h1' = 20 kJ/kg , s2' = 0.7366
h2' = 20 kJ/kg , s1' = 0.07
s2 = s1' + x(s2' - s1')
= 0.07 + x + (0.7366 – 0.07)
⇒ 0.67 = 0.07 + 0.6666 x⇒ x = 0.67 - 0.07 = 0.90 0.6666
h2 = h1' + x(h2' - h1')
= 20 + 0.9(180 – 20)
= 20 + 0.9 × 160 = 165 kJ/kg
Refrigerating effect per kg = h2 – h1 = 164 – 80 = 84 kJ/kg
Hence refrigerating effect for 0.025 kg/sec circulation = 84 × 0.025 = 2.1 kW
- In a vapour compression refrigeration system, liquids to suction heat exchanger is used to
-
View Hint View Answer Discuss in Forum
NA
Correct Option: C
NA
- Round the clock cooling of an apartment having a load of 300 MJ/day requires and air conditioning plant of capacity about
-
View Hint View Answer Discuss in Forum
Capacity= Q = 300 MJ/day
= 300 × 103 kJ / s 24 × 60 × 60
= 3.47 kW
1 ton = 3.5 kW∴ Q = 3.47 = 0.99 ton = 1 ton 3.5 Correct Option: A
Capacity= Q = 300 MJ/day
= 300 × 103 kJ / s 24 × 60 × 60
= 3.47 kW
1 ton = 3.5 kW∴ Q = 3.47 = 0.99 ton = 1 ton 3.5
- Dew point temperature of air at one atmospheric pressure (1.013 bar) is 18°C. The air dry bulb temperature is 30°C. The saturation pressure of water at 18°C and 30°C are 0.02062 bar and 0.04241 bar respectively. The specific heat of air and water vapour respeetively are 1.005 and 1.88 kJ/kgK and the latent heat of vaporization water of 0°C is 2500 kJ/kg. The specific humidity (kg/kg of dry air) and enthalpy (kJ/kg of dry air) of this moist air respectively, are
-
View Hint View Answer Discuss in Forum
Given: p = 1.013 bar, (pυ)tdp = 0.02062
Specific humidity = 0.622 Pυ p - Pυ = 0.622 × 0.02062 = 0.01291 kg/kg of dry air 1.013 - 0.02062
h = 1.022 td + w(hfgdp + 2.3tdp)
= 63.15 kJ/kg of dry air.
Alternative method
pε = Saturation pressure = 0.02062 bar
pa = Partial pressure of air = 0.04241 bar
td = Dry bulb temperature = 30º
tw = Wet bulb temperature = 18º
Specific humidity,W = pυ × 0.622 = 0.01292 kg/kg of dry air. p - pυ
Total enthalpy,
h = cpa td + whs + wc ps (td – tω)
= 1.005 × 30 + 0.0129 × 2500 + 0.01292 × 1.88(30 – 18)
= 63.15 kJ/kg of dry air.Correct Option: B
Given: p = 1.013 bar, (pυ)tdp = 0.02062
Specific humidity = 0.622 Pυ p - Pυ = 0.622 × 0.02062 = 0.01291 kg/kg of dry air 1.013 - 0.02062
h = 1.022 td + w(hfgdp + 2.3tdp)
= 63.15 kJ/kg of dry air.
Alternative method
pε = Saturation pressure = 0.02062 bar
pa = Partial pressure of air = 0.04241 bar
td = Dry bulb temperature = 30º
tw = Wet bulb temperature = 18º
Specific humidity,W = pυ × 0.622 = 0.01292 kg/kg of dry air. p - pυ
Total enthalpy,
h = cpa td + whs + wc ps (td – tω)
= 1.005 × 30 + 0.0129 × 2500 + 0.01292 × 1.88(30 – 18)
= 63.15 kJ/kg of dry air.
- A stream of moist air (mass flow rate = 10.1 kg/s) with humidity ratio of 0.01 kg/kg dry air mixes with a second stream of superheated water vapour flowing at 0.1 kg/s. Assuming proper and uniform mixing with no condensation, the humidity ratio of the final stream in (kg/kg dry air) is ______.
-
View Hint View Answer Discuss in Forum
ωnew = m1ω1 + m2ω2 m1 + m2 ⇒ 0.1 × 10.1 × + .1 × 1 = 0.02kg/kg dry air 10.1 + .1
Correct Option: A
ωnew = m1ω1 + m2ω2 m1 + m2 ⇒ 0.1 × 10.1 × + .1 × 1 = 0.02kg/kg dry air 10.1 + .1

