Refrigeration and Air-conditioning Miscellaneous
- The pressure, dry bulb temperature and relative humidity of air in a room are 1 bar, 30°C and 70%, respectively. If the saturated steam pressure at 30°C is 4.25 kPa, the specific humidity of the room air in kg of water vapour/kg dry air is
-
View Hint View Answer Discuss in Forum
RH = 0.7 = Pv and w = 0.622pv 4.25 100 - pv
Correct Option: C
RH = 0.7 = Pv and w = 0.622pv 4.25 100 - pv
- A room contains 35 kg of dry air and 0.5 kg of water vapor. The total pressure and temperature of air in the room are 100 kPa and 25°C respectively. Given that the saturation pressure for water at 25°C, is 3.17 kPa, the relative humidity of the air in the room is
-
View Hint View Answer Discuss in Forum
ma = 35 kg, mv = 0.5 kg
w = mv = 0.5 = 0.01428 mv 35 also w = 0.622 Pv Pa
Pt = 100 kPa∴ w = 0.622 × Pv Pt - Pv Now, 0.01428 = 0.622 × 
Pv 
Pt - Pv
Pv = 2.238 kPa
Relative humidityφ = Pv = 2.238 × 100 Ps 3.17
φ = 70.6% = 71%Correct Option: D
ma = 35 kg, mv = 0.5 kg
w = mv = 0.5 = 0.01428 mv 35 also w = 0.622 Pv Pa
Pt = 100 kPa∴ w = 0.622 × Pv Pt - Pv Now, 0.01428 = 0.622 × 
Pv 
Pt - Pv
Pv = 2.238 kPa
Relative humidityφ = Pv = 2.238 × 100 Ps 3.17
φ = 70.6% = 71%
- If a mass of moist air in an airtight vessel is heated to a higher temperature, then
-
View Hint View Answer Discuss in Forum
R.H. decreases

Correct Option: D
R.H. decreases

- A moist air sample has dry bulb temperature of 30°C and specific humidity of 11.5 g water vapour per kg dry air. Assume molecular weight of air as 28.93. If the saturation vapour pressure of water at 30°C is 4.24 kPa and the total pressure is 90 kPa, then the relative humidity (in%) of air sample is
-
View Hint View Answer Discuss in Forum
Given: DBT = 30°C, w = 11.5 g/kg
∴ Relative humidity= Specific humidity × Total pressure 0.622 saturation vapour pressure φ = w × Pa 0.622 Pvs φ = 11.5 × 90 = 0.385 0.622 × 1000 × 4.24
φ ≈ 38.5%Correct Option: B
Given: DBT = 30°C, w = 11.5 g/kg
∴ Relative humidity= Specific humidity × Total pressure 0.622 saturation vapour pressure φ = w × Pa 0.622 Pvs φ = 11.5 × 90 = 0.385 0.622 × 1000 × 4.24
φ ≈ 38.5%
- Moist air at a pressure of 100 kPa is compressed to 500 kPa and then cooled to 35°C in an aftercooler. The air at the entry to the aftercooler is unsaturated and becomes just saturated at the exit of the aftercooler. The saturation pressure of water at 35°C is 5.628 kPa. The partial pressure of water vapour (in kPa) in the moist air entering the compressor is closest to
-
View Hint View Answer Discuss in Forum
Assuming that compression is isentropic in air compressors, the process can be described on the T - s diagram. The process in the intercooler is constant pressure
p2 = p3
But p2 = 5p1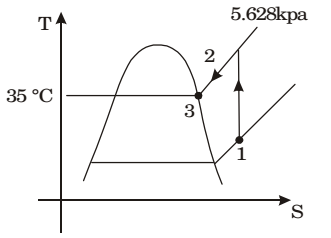
p1 = p2 = p3 5 5 p1 = 5.628 = 1.13kPa 5
Correct Option: B
Assuming that compression is isentropic in air compressors, the process can be described on the T - s diagram. The process in the intercooler is constant pressure
p2 = p3
But p2 = 5p1
p1 = p2 = p3 5 5 p1 = 5.628 = 1.13kPa 5

