Thermodynamics Miscellaneous
- In the figure shown, the system is a pure substance kept in a piston-cyllnder arrangement. The system is initially a twophase mixture containing 1 kg of liquid and 0.03 kg of vapour at a pressure of 100 kPa. Initially, the piston rests on a set of stops, a shown in the figure. A pressure of 200 kPa is required to exactly balance the weight of the piston and the outside atmospheric pressure. Heat transfer takes place into the system until its volume increases by 50%. Heat transfer to the system occurs in such a manner that the piston, where it is allowed to move, does so in a very slow (quasi-Static/quasi-equilibrium) process. The thermal reservoir from which heat is transferred to the system has a temperature of 400°C. Average temperature of the system boundary can be taken as 175°C. The heat transfer to the system is 1 kJ, during which its entropy increases by 10 J/K. Specific volumes of liquid (vf) and vapour (vg) phases, as well as values of saturation temperatures, are given in the table below.

Pressure Saturation Vf ( m3/Kg ) Vg( m3/Kg ) 100 100 0.001 0.1 200 200 0.0015 0.002
The work done by the system during the process is
-
View Hint View Answer Discuss in Forum
w = pdv
w = 200 (v2 – v1)
w = 200 (0.5 × v1)
v1 = 200 × 0.5 × 0.004
[w = 0.4 kJ]Correct Option: D
w = pdv
w = 200 (v2 – v1)
w = 200 (0.5 × v1)
v1 = 200 × 0.5 × 0.004
[w = 0.4 kJ]
- In the vicinity of the triple point, the vapour pressures of liquid and solid ammonia are respectively given by ln P= 15.16 – 3063/T and In P= 18.70 – 3754/T where P is in atmospheric and T is in kelvin. What is the temperature at the triple point?
-
View Hint View Answer Discuss in Forum
At triple point the vapour pressure of solid ammonia is equal to vapor pressure of liquid ammonia
-3063 = 18.7 - 3754 T T T = 3754 - 3036 = 195.2 K 18.7 - 15.16
Correct Option: C
At triple point the vapour pressure of solid ammonia is equal to vapor pressure of liquid ammonia
-3063 = 18.7 - 3754 T T T = 3754 - 3036 = 195.2 K 18.7 - 15.16
- Availability of a system at any given state is
-
View Hint View Answer Discuss in Forum
Availability of system of any given state is when no maximum useful work obtainable as the system goes to dead state.
Correct Option: D
Availability of system of any given state is when no maximum useful work obtainable as the system goes to dead state.
- A solar collector receiving solar radiation at the rate of 0.6 kW/m2 transforms it to the internal energy of a fluid at an overall efficiency of 50%. The fluid heated to 350 K is used to run a heat engine which rejects heat at 313 K. If the heat engine is to deliver 2.5 kW power, the minimum area of the solar collector required would be
-
View Hint View Answer Discuss in Forum
10. Given; Receiving solar radiation at the rate of 0.6 kW/m2
Internal energy of fluid after absorbing solar radiation = 0.6 × 1 kW / m2 = 0.3 kW / m2 2 ηengine = 1 - 315 = 0.1 350 0.1 = W Q1 ∴ Q1 = 2.5 = 25 k W 0.1
Let A be minimum area of collector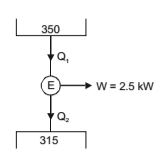
∴ Q1 = 0.3 × A or 25 kW = 0.3 kW/m2or A = 25 = 83.33 m2 0.3
Correct Option: A
10. Given; Receiving solar radiation at the rate of 0.6 kW/m2
Internal energy of fluid after absorbing solar radiation = 0.6 × 1 kW / m2 = 0.3 kW / m2 2 ηengine = 1 - 315 = 0.1 350 0.1 = W Q1 ∴ Q1 = 2.5 = 25 k W 0.1
Let A be minimum area of collector
∴ Q1 = 0.3 × A or 25 kW = 0.3 kW/m2or A = 25 = 83.33 m2 0.3
- A heat reservoir at 900 K is brought into contact with the ambient at 300 K for a short time. During this period 9000 kJ of heat is lost by the heat reservoir. The total loss in availability due to this process is
-
View Hint View Answer Discuss in Forum
Entropy change for hot reservoir ∆Sh = Q = -9000 = -10 kJ/ k T 900 Energy gain in cold reservoir ∆Sc = Q = -9000 = +30 kJ/ k T 300
Loss in availability = T0[∆Sc + ∆Sh]
⇒ Loss in availability = 300(30 – 10) = 300(20) = 6000 kJCorrect Option: C
Entropy change for hot reservoir ∆Sh = Q = -9000 = -10 kJ/ k T 900 Energy gain in cold reservoir ∆Sc = Q = -9000 = +30 kJ/ k T 300
Loss in availability = T0[∆Sc + ∆Sh]
⇒ Loss in availability = 300(30 – 10) = 300(20) = 6000 kJ

