-
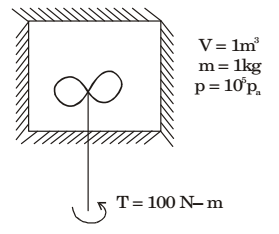
A well insulated rigid container of volume 1 m3 contains 1.0 kg of an ideal gas [cp = 1000 J/(kgK) and cv = 800 J/(kgK)] at a pressure of 105 Pa. A stirrer is rotated at constant rpm in the container for 1000 rotations and the applied torque is 100 Nm. The final temperature of the gas (in K) is______.
-
- 1195.4 K
- 1295 K
- 1295.4 K
- 2295.4 K
Correct Option: C
| T1 = | = | |||
| mR | m(cp - cv) |
T1 ⇒ 500k
w = 2πNT = - 2π × 105 J
Q = U + W (insulated)
W = –U
U = 2π × 105
Cv (T – 500) = 2π × 105
T = 1295.4 K

