-
An ideal gas undergoes a reversible process in which the pressure varies linearly with volume. The conditions at the start (subscript 1) and at the end (subscript 2) of the process with usual notation are: p1 = 100 kPa, V1 = 0.2 m3 and p2 = 200 kPa, V2 = 0.1 m3 and the gas constant, R = 0.275 kJ/kgK. The magnitude of the work required for the process (in kJ) is______.
-
- 16 kJ
- 25 kJ
- 18 kJ
- 15 kJ
Correct Option: D
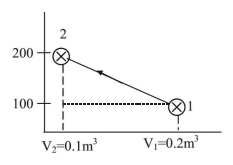
| W2 = | (P1 + P2)(V1 - V2) | 2 |
| = | (100 + 200)(0.2 - 0.1) | 2 |
15 kJ

