-
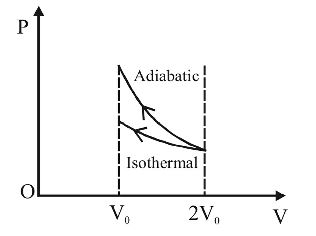
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then :
-
- Compressing the gas isothermally will require more work to be done.
- Compressing the gas through adiabatic process will require more work to be done.
- Compressing the gas isothermally or adiabatically will require the same amount of work.
- Which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
Correct Option: B
Wext = negative of area with volume-axis
W(adiabatic) > W(isothermal)