-
Figure below shows two paths that may be taken by a gas to go from a state A to a state C.
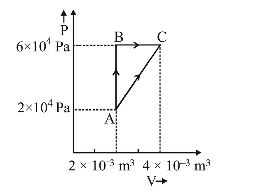
In process AB, 400 J of heat is added to the system and in process BC, 100 J of heat is added to the system. The heat absorbed by the system in the process AC will be
-
- 500 J
- 460 J
- 300 J
- 380 J
Correct Option: B
In cyclic process ABCA
Qcycle = Wcycle
| QAB + QBC + QCA = ar. of ∆ABC + 400 + 100 + QC→A = | (2 × 10-3)(4 × 104) | |
| 2 |
⇒QC → A = – 460 J
⇒QA → C = + 460 J

