-
A radio isotope ‘X’ with a half life 1.4 × 109 years decays to ‘Y’ which is stable. A sample of the rock from a cave was found to contain ‘X’ and ‘Y’ in the ratio 1 : 7. The age of the rock is :
-
- 1.96 × 109 years
- 3.92 × 109 years
- 4.20 × 109 years
- 8.40 × 109 years
Correct Option: C
| As | = | (Given) | ||
| Ny | 7 |
| ⇒ | = | |||
| Nx + Ny | 8 |
| = | 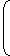 | 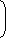 | 3 | 2 |
Therefore, age of the rock
t = 3T1/2 = 3 × 1.4 × 109 yrs = 4.2 × 109 yrs.

