-
Figure below shows a reversible heat engine ER having heat interactions with three constant temperature systems. Calculate the thermal efficiency of the heat engine
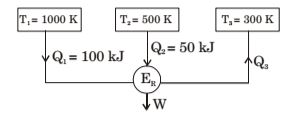
-
- 60%
- 30%
- 20%
- 5%
Correct Option: A
Q1 + Q2 - Q3 = W .... (i)
| + | - | = 0 ...... (ii) | ||||
| 1000 | 500 | 300 |
From equation (ii) , we have
| + | - | = 0 | ||||
| 1000 | 500 | 300 |
⇒ Q3 = 60 kJ
W ⇒ 100 + 50 - 60 = 90 kJ
| η = | = | = 60% | ||
| Q1 + Q2 | 150 |

