-
A reversible heat engine receives 2 kJ of heat from a reservoir at 1000 K and a certain amount of heat from a reservoir at 800 K. It rejects 1 kJ of heat to a reservoir at 400 K. The net work output (in kJ) of the cycle is
-
- 0.8
- 1.0
- 1.4
- 2.0
Correct Option: C
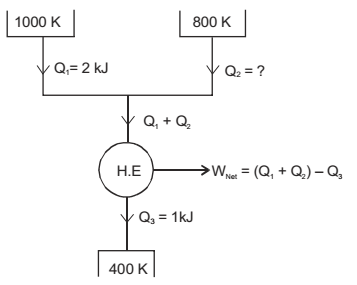
We know that for reversible heat engine, change in entropy is always zero
That is ∆S = 0
| - |  | + |  | = 0 | T3 | T1 | T2 |
| - | - | = 0 | ||||
| 400 | 1000 | 800 |
Q2 = 0.4 kJ
WNet = (Q1 + Q2) – Q3 = (2 + 0.4) – 1 = 1.4 kJ.

