-
A Carnot engine (CE-1) works between two temperature reservoirs A and B, where TA = 900 K and TB = 500 K. A second Carnot engine (CE-2) works between temperature reservoirs B and C, where Tc = 300 K. In each cycle of CE-1 and CE-2, all the heat rejected by CE-1 to reservoir B is used by CE-2. For one cycle of operation, if the net Q absorbed by CE-1 from reservoir A is 150 MJ, the net heat rejected to reservoir C by CE-2 (in MJ) is ______.
-
- 50
- 25
- 40
- 30
Correct Option: A
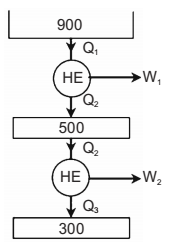
| η1 = | = | = 4.44 | ||
| T1 | 900 |
Q2 = (1 – η1) × Q1 = 53.33 MJ
| η2 = 1 - | = 1 - | = 0.4 | ||
| T2 | 500 |
Q3 = (1 – η2) × Q2 = 50 MJ

