-
A steel billet of 2000 kg mass is to be cooled from 1250 K to 450 K. The heat released during this process is to be used as a source of energy. The ambient temperature is 303 K and specific heat of steel is 0.5 kJ/kgK. The available energy of this billet is
-
- 490.44 MJ
- 30.95 MJ
- 10.35 MJ
- 0.10 MJ
Correct Option: A
Heat lost by steel = (0.5)(2000)(450 – 1250) Gained = 800MJ
| ∆SLost by steel = (2000) (0.5)) ln | 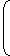 | 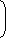 | 1250 |
⇒ –1.021 MJ
(∆S) gained = 1.021MJ
AE (W) = a – T0 dS
= 800 – (303) (1.021)
= 490.7 MJ

