-
The empirical formula for biomass of an unknown organism is CH1.8 O0.5 N0.2. To grow this organism, ethanol (C2H5OH) and ammonia are used as carbon and nitrogen sources, respectively. Assume no product formation other than biomass. To produce 1 mole of biomass from 1 mole of ethanol, the number of moles of oxygen required will be ___________.
-
- 1.9 to 2.0
- 5.9 to 7.2
- 1.0 to 1.5
- None of the above
Correct Option: A
The equation for biomass production is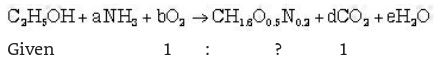
Now making elemental balance on both sides.
LHS C = 2
N = a i.e. a = 0.2
RHS C =1 + d = 2 i.e. d = 1
N = 0.2
Now, putting value of a & d in equation (i)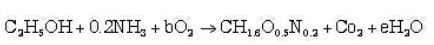
Now, H balance
LHS H = 6 + 0.6 = 6.6
RHS H = 1.8 + 2e = 6.6
∴ e = 2.4
and finally O2 balance
LHS
0 = 1 + 2b
RHS
0 = 0.5 + 2 + 2.4 (i.e. e = 2.4) = 4.9
hence1 + 2b = 4.9
∴ 2b = 3.9
∴ b =1.95
∴ Final equation
C2H5OH + 0.2 NH3 + 1.95O2 → CH1.2O0.5N0.2 + CO2 + 2.4 H2O

